

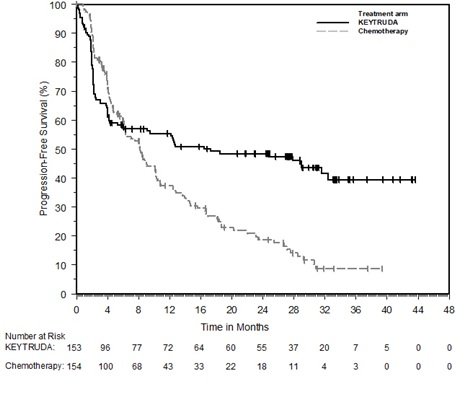
Severe treatment-related adverse events (AEs grade 3 or greater) were also less common with pembrolizumab than chemotherapy (22% vs 66%). The data presented have the potential to change the standard of care.” “Now, in studies like this one, we are starting to see significant efficacy for immunotherapies as first-line treatment for advanced cancers with specific genetic signatures, in this case metastatic colorectal carcinoma with microsatellite instability high/mismatch repair deficient mutations. Burris III, MD, FACP, FASCO, said in the release. “Immunotherapies like pembrolizumab have proved to be effective as second-line treatments for advanced disease,” ASCOâ¯President Howard A. Response with pembrolizumab was also longer lasting, with 83% of patients having a response longer than 2 years, compared with 35% of patients receiving chemotherapy. In comparison, 3.9%, 29.2% and 42.2% of patients, respectively, receiving chemotherapy had complete response, partial response, and stable disease. Moreover, 11% of patients receiving pembrolizumab had a complete response, 32.7% had a partial response, and 30.9% had stable disease. The ORR was also better with pembrolizumab as well, with 43.8% seeing a reduction in tumor size compared with 33.1% for chemotherapy. Key secondary endpoints included overall response rate (ORR) and safety.Īt 12- and 24-months follow up, PFS was 55.3% and 48.3%, respectively, with pembrolizumab versus 37.3% and 18.6% with chemotherapy. PFS and overall survival (OS) were the primary endpoints for the trial. The investigators had their choice of mFOLFOX6 (5-fluorouracil, leucovorin, and oxaliplatin), mFOLFOX6 + bevacizumab (Avastin), mFOLFOX6 + cetuximab (Erbitux), FOLFIRI (leucovorin, 5-fluorouracil, and irinotecan), FOLFIRI + bevacizumab, or FOLFIRI + cetuximab. Patients were randomly assigned to receive either first-line pembrolizumab for up to 2 years or the investigator’s choice of 6 different standard chemotherapy regimens, selected prior to randomization.
Pembrolizumab works by blocking the activity of PD-1 receptors, allowing the immune system to attack cancer cells.Īs of February 19, 2020, the time of data cutoff for this interim analysis, the phase III KEYNOTE-177 study included 307 patients with MSI-H or dMMR mCRC. In phase II studies, pembrolizumab demonstrated durable antitumor activity with an acceptable safety profile in previously treated MSI-H mCRC. The presence of MSI-H or dMMR tumors is generally associated with decreased survival rates, and patients with MSI-H or dMMR metastatic disease also tend to be less responsive to conventional chemotherapy. Overall, patients with MSI-H CRC represent 5% of all patients with mCRC. This randomized study demonstrates a huge benefit in first line with pembrolizumab and should be the new standard of care.” 2 “Pembrolizumab works in non-randomized studies in this group of patients with advanced disease.
#Keynote 177 colorectal trial#
“These long-awaited trial results will change clinical practice,” lead author Thierry André, MD, of the Sorbonne Université and Hôpital Saint Antoine in Paris, said in an ASCO-issued press release. The randomized, open-label, phase III trial, presented during a 2020 ASCO Virtual Scientific Program press briefing, is the first in which pembrolizumab has been shown to benefit these patients when used as a front-line therapy. The phase III KEYNOTE-177 study demonstrated that front-line therapy with the immune checkpoint inhibitor pembrolizumab (Keytruda) doubled progression-free survival (PFS) versus standard of care chemotherapy in patients with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) metastatic colorectal cancer (mCRC).


 0 kommentar(er)
0 kommentar(er)
